Chicken pox and its complications
Varicella-zoster virus (VZV)
- Causes- primary, latent, and recurrent infections.
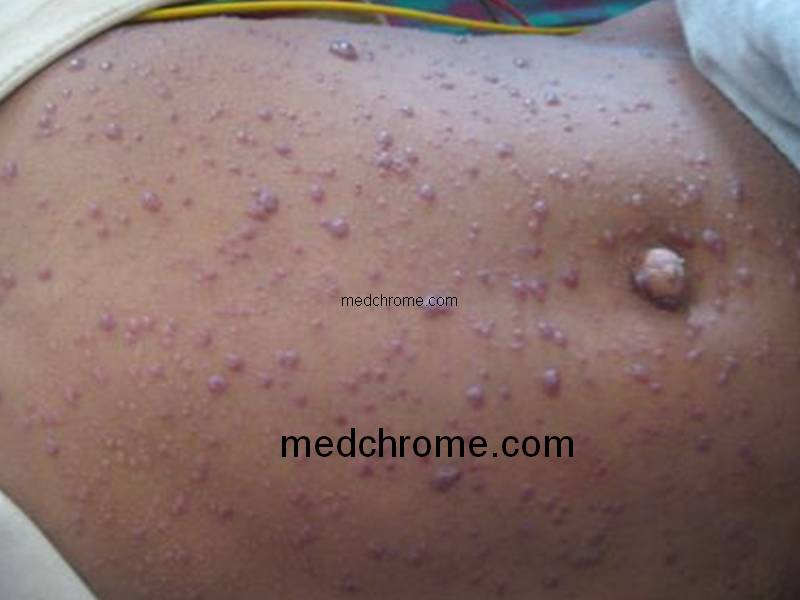
- Primary infection – Varicella (chickenpox)
- A lifelong latent infection of sensory ganglion neurons.
- Reactivation of the latent infection – Herpes zoster (shingles).
Epidemiology
Most children are infected by 15 yr of age, with fewer than 5% of adults remaining susceptible.Within households, transmission of VZV to susceptible individuals occurs at a rate of 65-86%
Infectivity : 24-48 hr before the rash is evident and until vesicles are crusted, usually 3-7 days after onset of rash.
Transmission: close, direct contact
Incubation Period ~14 days (10-21 days).
Fenner and Grose proposed that chickenpox begins with replication of the virus at sites of contact and is followed à primary viremia which establishes replication in the reticuloendothelial system. A secondary viremia occurs after about 1 week, which disseminates the infection to the skin, causing the familiar rash to appear. As the acute disease abates, the virus establishes latency in sensory ganglia. Reactivation of latent infection causes zoster or “shingles,”à pain and a vesicular rash in the distribution of a dermatome. Replication of the virus in the ganglia is destructive to ganglion cells, which may account for the pain.
Recent – Tonsilsà CD4+ T cells to skin
Pathogenesis
Progressive varicella
Visceral organ involvement, coagulopathy, severe hemorrhage, and continued vesicular lesion development.
Severe abdominal pain (mesenteric lymph nodes or the liver involvement) or
the appearance of hemorrhagic vesicles in otherwise healthy adolescents and adults, immunocompromised children, pregnant women, and newborns, may herald severe disease.
The risk for progressive varicella is highest in children with
–congenital cellular immune deficiency disorders
–those with malignancy, particularly if chemotherapy was given during the incubation period and the absolute lymphocyte count is <500 cells/mm3.
Varicella-related deaths usually occur within 3 days after the diagnosis of varicella pneumonia.Unusual clinical findings of varicella, including lesions that develop a unique hyperkeratotic appearance and continued new lesion formation for weeks or months, have been described in children with untreated, late stage HIV infection.

Investigation of chickenpox
Laboratory evaluation not necessary for the diagnosis or management of healthy children with varicella or herpes zoster -Clinical Diagnosis.
Molecular techniques (PCR) or
Virus isolation from fluid/cells from the base of the vesicular lesion.
Associated Lab findings-
Leukopenia (1st 72 hours after onset of rash; it is followed by a relative and absolute lymphocytosis)
LFT – usually (75%) mildly elevated.
CSF- mild lymphocytic pleocytosis and a slight to moderate increase in protein content ,glucose concentration is usually normal. ( patients with neurological complications)
Diagnosis
Rapid laboratory diagnosis-
Direct fluorescence assay (DFA) of cells from cutaneous lesions (vesicular fluid)
PCR amplification testing (vesicular fluid, crusts) .
Tzanck smear- poor sensitivity and do not differentiate VZV from HSV infections.
Tissue Culture methods- virus may take days to weeks to grow.
VZV antibody test – IgG can be detected by several methods 4 fold or greater rise in IgG – confirmatory of acute infection.
VZV IgG antibody tests -valuable to determine the immune status of individuals
Strain identification (genotyping) can distinguish wild-type VZV from the vaccine strain
Complications
Varicella hepatitis – relatively common but rarely clinically symptomatic.
Thrombocytopenia 1-2% – transient petechiae. Purpura, hemorrhagic vesicles, hematuria, and gastrointestinal bleeding are rare complications that may have serious consequences.
Other complications of varicella, some of them rare, include
- cerebellar ataxia
- encephalitis
- pneumonia
- nephritis
- nephrotic syndrome
- hemolytic-uremic syndrome,
- arthritis
- Myocarditis,pericarditis
- pancreatitis
- orchitis.
Hemorrhagic complications – pulmonary and gastrointestinal bleeding, intra-cerebral haemorrhage, disseminated intravascular coagulation.
Evidence of bleeding,-easy bruising, hemorrhagic skin lesions, Bleeding gums, or hemoptysis
Bacterial Infections –
- Group A streptococci and S. aureus,- 5% of children with varicella
- An early manifestation – erythema of the base of a new vesicle.
- Recrudescence of fever 3-4 days after the initial exanthem may also herald it.
- The more invasive infections, such as varicella gangrenosa, bacterial sepsis, pneumonia, arthritis, osteomyelitis, cellulitis, and necrotizing fasciitis.
- Toxic shock syndrome
Indicators of severe disease in acute varicella infection
1.Respiratory symptoms (clinical respiratory signs are often absent).
2. Densely cropping vesicles.
3. Haemorrhagic rash.
4. Bleeding (e.g. from gums, hemoptysis, GI bleeding).
5. Any neurological changes (cerebellar signs, encephalopathy).
6. Persisting fever with new vesicles >6 days after onset.
Encephalitis and Cerebellar Ataxia
Encephalitis (1/50,000 cases in unvaccinated children)-Nuchal rigidity, altered consciousness, and seizures .
Acute cerebellar ataxia (1/4,000 cases in unvaccinated children)- gradual onset of gait disturbance, nystagmus, and slurred speech.
morbidity from CNS complications is highest among patients younger than 5 yr or older than 20 yr.
Neurologic symptoms usually begin 2-6 days after the onset of the rash but may occur during the IP or after resolution of the rash.
Clinical recovery is typically rapid, occurring within 24-72 hr, and is usually complete.
Although severe hemorrhagic encephalitis, analogous to that caused by herpes simplex virus, is very rare in children with varicella, the consequences are similar to those of herpes encephalitis.
Reye syndrome
Pneumonia
Varicella pneumonia- severe complication
Accounts for increased morbidity and mortality in adults and other high-risk populations,
pneumonia may also complicate varicella in young children.
Respiratory symptoms, which may include cough, dyspnea, cyanosis, pleuritic chest pain, and hemoptysis, usually begin within 1-6 days after the onset of the rash.
Smoking- risk factor
The frequency of varicella pneumonia may be greater in the parturient.
Treatment
Antiviral treatment modifies the course of both varicella and herpes zoster.
Foscarnet -the only drug available for the treatment of acyclovir-resistant VZV infections.
Acyclovir- only drug available in liquid formulation that is licensed for pediatric use .
Good safety profile and efficacy in the treatment of varicella, treatment of all children, adolescents, and adults with varicella is acceptable.
However, acyclovir therapy is not recommended routinely by the American Academy of Pediatrics for treatment of uncomplicated varicella in the otherwise healthy child because of the marginal benefit, the cost of the drug, and the low risk for complications of varicella.
Oral therapy with acyclovir (20 mg/kg/dose, maximum 800 mg/dose) given as 4 doses/day for 5 days can be used to treat uncomplicated varicella in at risk patients.
To be most effective- preferably within 24 hr of the onset of the exanthem.
Less clinical benefit after 72 hr after onset of the exanthem.
Intravenous therapy – indicated for severe disease and for varicella in immunocompromised patients (even if begun 72 hr after onset of rash).
Acyclovir has been used to treat varicella in pregnant women; its safety for the fetus has not been established.
Some experts recommend the use of famciclovir or valacyclovir -better absorbed by the oral route than acyclovir.
Any patient who has signs of disseminated VZV, including pneumonia, severe hepatitis, thrombocytopenia, or encephalitis, should receive immediate treatment.
IV acyclovir (500 mg/m2 every 8 hr IV) therapy initiated within 72 hr of development of initial symptoms decreases the likelihood of progressive varicella and visceral dissemination in high-risk patients.
Treatment is continued for 7–10 days or until no new lesions have appeared for 48 hr.
Acyclovir-resistant VZV has been identified in children infected with HIV. These children may be treated with intravenous foscarnet, 120 mg/kg/day divided every 8 hr for up to 3 wk.
Prognosis
Primary varicella has a mortality rate of 2-3/100,000 cases, with the lowest case fatality rates among children 1-9 yr of age (~1 death per 100,000 cases).
Compared with these age groups, infants have a 4 times greater risk of dying and adults have a 25 times greater risk of dying.
The mortality rate of untreated primary infection is 7-14% in immunocompromised children and may approach 50% in untreated adults with pneumonia.
Prevention
VZV transmission is difficult to prevent.
Infection control practices, including caring for infected patients in isolation rooms with filtered air systems, are essential.
All health care workers should have evidence of varicella immunity .
Unvaccinated health care workers without other evidence of immunity who have had a close exposure to VZV should be furloughed for days 8-21 after exposure because they are potentially infectious during this period.
EVIDENCE OF IMMUNITY TO VARICELLA
Evidence of immunity to varicella consists of any of the following:
Documentation of age-appropriate vaccination with a varicella vaccine:
Preschool-aged children (i.e., aged >12 mo): 1 dose
School-aged children, adolescents, and adults: 2 doses*
Laboratory evidence of immunity[†] or laboratory confirmation of disease
Diagnosis or verification of a history of varicella disease by a health-care provider
Diagnosis or verification of a history of herpes zoster by a health-care provider
For children who received their first dose at age <13 yr and for whom the interval between the 2 doses was ≥28 days, the second dose is considered valid.
Vaccine
Varicella vaccine contains live, attenuated VZV (Oka strain) and is indicated for subcutaneous administration.
Recommended for routine administration as a 2-dose regimen to healthy children at ages 12-15 mo and 4-6 yr.
Catch-up vaccination with the 2nd dose- recommended for children and adolescents who received only 1 dose.
Vaccination with 2 doses is recommended for all persons without evidence of immunity.
The minimum recommended interval between the two doses is 3 mo for persons ≤12 yr of age and 4 wk for older children, adolescents, and adults.
Administration of varicella vaccine within 4 wk of measles-mumps-rubella (MMR) vaccine has been associated with a higher risk for breakthrough disease; therefore, it is recommended that the vaccines either be administered simultaneously at different sites or be given at least 4 wk apart.
Contraindicated – pregnant women and persons with CMI deficiencies( leukemia, lymphoma, and other malignant neoplasms affecting the bone marrow or lymphatic systems)
Compassionate-use protocols are available for immunization of children with leukemia in remission.
The vaccine should be considered for HIV-infected children with a CD4+ T-lymphocyte percentage ≥15%. These children should receive 2 doses of vaccine, 3 mo apart.
Children with isolated humoral immunodeficiencies may receive varicella vaccine.
Vaccine-associated Adverse Events
Varicella vaccine is safe and well tolerated.
The incidence of injection site complaints observed ≤3 days after vaccination was slightly higher after dose 2 (25%) than after dose 1 (22%).
Mild vaccine-associated varicelliform rash 1-3% of healthy vaccinees, consisting of 6-10 papular-vesicular, erythematous lesions with peak occurrence 8-21 days after vaccination.
Transmission of vaccine virus to susceptible contacts is a very rare occurrence.
Varicella in Vaccinated Individuals (“Breakthrough Varicella”)
One dose of varicella vaccine is >97% effective in preventing severe varicella and is 85% (median; range 44-100%) effective in preventing all disease after exposure to wild-type VZV. Breakthrough disease is varicella that occurs in a person vaccinated >42 days before rash onset and is caused by wild-type VZV. Rash occurring 14-42 days after vaccination was due to either wild or vaccine strains, reflecting breakthrough varicella or vaccine-associated rash, respectively. The rash in breakthrough disease – atypical and predominantly maculopapular, vesicles are seen less commonly, and the illness is most commonly mild with <50 lesions, shorter duration of rash, fewer complications, and little or no fever.
25-30% of breakthrough cases are not mild, with clinical features more similar to those of wild-type infection.
Overall less contagious than wild-type infections
–<50 lesions- 1/3 as contagious as unvaccinated cases
–≥50 lesions are as contagious as wild-type cases.
Fewer studies have evaluated the performance of the 2-dose varicella vaccine regimen. One clinical trial estimated the 2-dose vaccine effectiveness for preventing all disease at 98%. Breakthrough cases have been reported among 2-dose vaccinees.
Postexposure Prophylaxis
Vaccine given to healthy children within 3-5 days after exposure (as soon as possible is preferred) -effective in preventing or modifying disease.
Varicella vaccine is now recommended for postexposure use and for outbreak control. High-titer anti-VZV immune globulin as postexposure prophylaxis is recommended for immunocompromised children, pregnant women, and newborns exposed to varicella.. The recommended dose is 1 vial (125 units) for each 10-kg increment of body weight (maximum 625 units) given IM within 96 hr after exposure.
Although licensed pooled immune globulin intravenous (IGIV) preparations contain antivaricella antibodies, the titer varies from lot to lot.
The recommended dose of IGIV for postexposure prophylaxis is 400 mg/kg administered once within 96 hr of exposure.
Immunocompromised patients who have received high-dose IGIV (100-400 mg/kg) for other indications within 2-3 wk before VZV exposure – expected to have serum antibodies to VZV.
IMPORTANT POINTS
1.VZV infection is especially severe in patients who are immunodeficient, and cell-mediated immunity appears to be an important factor in limiting infection and in adults.
2.Bacterial superinfection is the most common complication of varicella and can be severe, particularly if group A Streptococcus is involved
3. Varicella zoster immune globulin (VZIG) is for prevention, not treatment, of disease. It must be given as soon as possible after exposure to the virus.
4. Varicella vaccine is safe and immunogenic, and its routine use should be advocated by all clinicians.
References
- Nelson Text Book of Pediatrics 19th Edition
- OP Ghai- 6th edition
- Varicella Zoster DOI: Pediatrics in Review 1998;19;62 Randall G. Fisher and Kathryn M. Edwards
- Varicella Zoster Infection DOI: 10.1542/pir.29-1-5Pediatrics in Review 2008;29;5 Anne A. Gershon
- Varicella-Zoster Virus- ANN M. ARVIN*Departments of Pediatrics and Microbiology/Immunology, Stanford University School of Medicine, Stanford, California 94305-5119
- Fatal hemorrhagic chickenpox in a case of acute promyelocytic leukaemia Asher Ahmed Mashhood, Nuzhat Salamat* Department of Dermatology, Combined Military Hospital Multan
- Chickenpox in adults e Clinical management A.J. Tunbridge a,*, J. Breuer b, K.J.M. Jeffery c, on behalf of the British Infection Society