Gullian Barre Syndrome – Post-infectious demyelinating polyneuropathy
Definition
Guillain-Barre syndrome is a postinfectious polyneuropathy involving mainly motor but sometimes also sensory and autonomic nerves. Guillain-Barré syndrome is an acute demyelinating disorder of the peripheral nervous system that results from an aberrant immune response directed at peripheral nervesThe pathogenesis of GBS is believed to be an abnormal immune response to an antecedent event, most commonly an infection. (Nelson Textbook of Pediatrics 18th Edition )
Epidemiology
Occurs year round and in all age groups.
Adults are affected more commonly than children.Males are more likely to be affected than females.
The overall incidence- 0.4 to 2.4 cases per 100,000 per year.
The incidence in children is lower, with estimates between 0.4 and 1.3 cases per 100,000 per year.
can occur at any age ,rare < 2 years. (Pediatrics in Review 2012;33;164 Beth A. Rosen )
History of AIDP/Guillain Barre Syndrome
First described by the French physician Jean Landry in 1859. In 1916, Georges Guillain, Jean Alexandre Barré, and André Strohl diagnosed two soldiers with the illness. Discovered the key diagnostic abnormality of increased CSF protein but normal cell count.
Etiology
About two-thirds of people who develop GBS symptoms do so several days (around 10 days) or weeks after they have been sick with a diarrhea or respiratory illness Many viruses and bacteria have been implicated, including Cytomegalovirus Epstein- Barr virus Haemophilus influenzae herpes simplex virus Mycoplasma.
The strongest association is with Campylobacter jejuni, which is believed to be the cause of 20% to 30% of GBS in the United States and Europe and a higher percentage of the AMAN cases seen in China and South America. Vaccines also have been implicated as a triggering factor. Some evidence suggests that the “swine flu” vaccine used in the 1976 influenza season led to an increased incidence of GBS.
There are no data linking other vaccines, including diphtheria-tetanus-acellular pertussis and measles-mumps-rubella, to GBS. Older-type rabies vaccine, prepared in nervous system tissue is implicated as a trigger of GBS in developing countries (where it is still used) may be due to immunization against neural antigens The number of GBS cases increased in both children and adults in Finland in 1985 after mass vaccination with oral polio Malignant lymphoma, liver cirrhosis, thyroid disorders (hyperthyroidism, Hashimoto thyroiditis), SLE, HIV positive, have been linked to Guillain-Barre syndrome
Pathogenesis
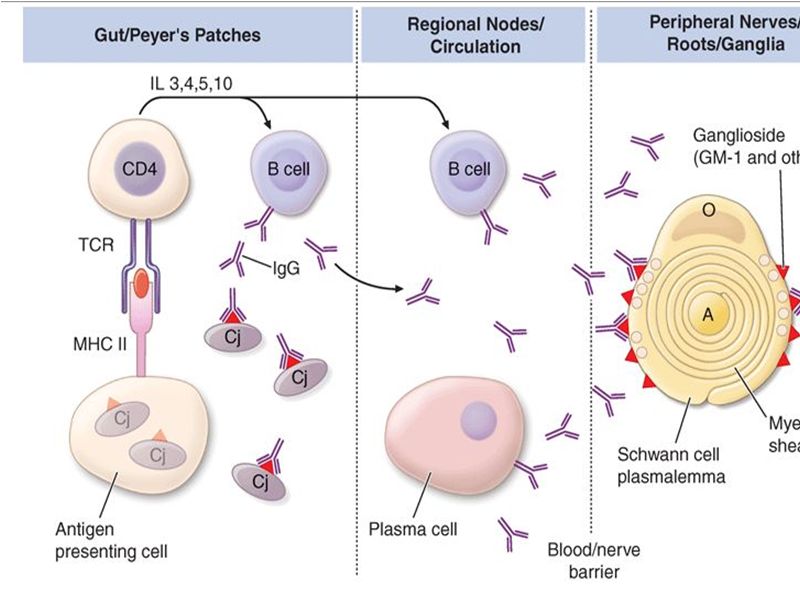
The acquired nature, the response to immunologic therapy and the pathology all suggest – GBS is an immune-mediated disease precise mechanism of the immunologic injury is unclear. A previous gastrointestinal infection with Campylobacter jejuni is associated with a more severe form of GBS Further evidence of immune-mediated disease is that peripheral nerve injury can be transferred passively from sera of patients who have GBS to laboratory animals. Experimental allergic neuritis (EAN) against P2 protein similar to AIDP.
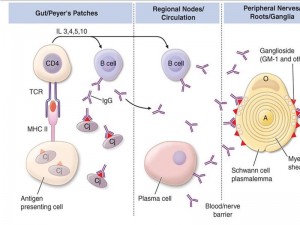
Multifocal areas of inflammation and demyelination with cellular infiltration of macrophages and lymphocytes In demyelinating form – macrophages penetrate the basement membrane of Schwann cells and strip away the myelin lamellae, leaving the axon exposed The focal demyelination causes conduction block, one of the electrodiagnostic features of the disease Intense inflammation at the junction of the dorsal and ventral roots leads to breakdown in the blood-brain barrier and the transudation of plasma proteins into the cerebrospinal fluid (CSF), Thus resulting characteristic albuminocytologic dissociation of the CSF Pathogenesis of GBS
The inflammation is more intense in the proximal nerve roots of the lumbar and brachial plexuses, but can involve the peripheral nerves, cranial nerves, dorsal roots, dorsal root ganglia, and the sympathetic chain. In summary, GBS is Caused by infection induced aberrant immune response that damages peripheral nerves Key factors in Pathogenesis Some key factors have been identified in the process Antiganglioside antibodies: Serum antibodies to various gangliosides in peripheral nerves have been found including GM1 GM1b GM2 GD1b GD2 GD3. Antibodies are specific to defined subgroup of GBS. Gangliosides GM1 GD1a with pure motor, GD3 GT1a with ophthalmoplegia and MFS Gangliosides: Complex glycosphingolipids that contain one or more sialic acid residues, cell-cell interactions , modulation of receptor Exposed on the plasma membrane of cells, rendering them susceptible to an antibody-mediated attack. Lancet neurology 2008; 7: 939-50
Key factors in Pathogenesis
Molecular mimicry and cross reactivity C. jejuni isolate from patients express lipooligosaccharides that mimic carbohydrate of gangliosides The type of ganglioside mimicry in C jejuni seems to determine specificity of antiganglioside antibody and associated variants of GBS. Molecular mimicry and cross reactivity have also been found in some infection with H influenzae
Complement activation Local complement activation occurs at the site of nerve damage Host factors Fewer than 1 in 1000 patients with C jejuni will develop GBS Epidemics have not occurred even in ganglioside mimicking variant of C jejuni Host factors might influence susceptibility or extent of nerve damage and outcome No association with HLA or single nucleotide polymorphism
Clinical presentations Symptoms :
Rapidly progressing weakness is core clinical feature of GBS First noticed as rubbery legs, accompanied by tingling dysesthesias in the extremities Legs are usually more affected than the arms, and facial diparesis is present in 50% of affected individuals, mostly symmetrical, asymmetry in 9 % of cases Maximum weakness is reached within 4 weeks but most patients within 2 weeks Then have plateau phase of varying duration days-weeks –months followed by recovery phase of varying duration.
Landry ascending paralysis: Weakness beginning in the lower extremities, progressing to trunk, upper extremities and finally the bulbar muscles
In cases with abrupt onset: pain and tenderness in the muscles is common, with Parasthesias, deep muscle pain, dysesthetic pain in extremities Weakness may progress to inability to walk and later flaccid tetraplegia Some young patients have signs of viral meningitis or meningoencephalitis.
Extraocular muscle involvement is rare
Miller fischer syndrome: External ophthalmoplegia Urinary incontinence or retention of urine is seen in around 25% cases and is usually transient (D/d from transverse myelitis)
About 33% of patients are mildly affected(are able to walk) Among severely affected patients (who are admitted and are unable to walk), 25% need artificial ventilation primarily because of weakness of respiratory muscles 20% of severely affected patients remain unable to walk after 6 months despite treatment including IV Ig or PE Facial nerve palsy is most common cranial nerve involvement in GBS.
Other cranial nerves may be affected Bickerstaff brainstem encephalitis starts with cranial or peripheral nerve involvement can progress to severe disturbance of consciousness and coma.
Clinical presentations Signs :
Conscious , may have respiratory distress, Heart Rate: normal, may have profound bradycardia or asystole but sometimes tachycardia Blood pressure: postural drop, hypertension, hypotension For this reason cardiac monitoring is must.
Fever if present points towards other diagnosis
CNS : Cranial nerve involvement, lower motor neuron type of lesion, flaccid paralysis with areflexia early in disease process, papilloedema Sometime Deep tendon reflexes may be preserved till late
Cerebellar signs : ataxia
Differential diagnosis of GBS (Acute Flaccid Paralysis)
Epidemiologically : Poliomyelitis, Transverse myelitis, Traumatic neuritis
Topographically :
Intracranial/spinal cord abnormalities
- Brainstem encephalitis
- tumors
- transverse myelitis
- cord compression
- epidural abscess
Anterior horn cell abnormalities-
- Poliomyelitis,
- West Nile virus
Spinal nerve root abnormalities
- Compression,
- inflammation (eg, cytomegalovirus),
- leptomeningeal malignancy
Peripheral nerve abnormalities
- CIDP (Chronic inflammatory demyelinating polyneuropathy)
- Critical illness polyneuropathy,
- Vasculitis Diphtheria
- Vitamin B1 deficiency (beri-beri),
- heavy metal or drug intoxication eg: Vincristine, Glue sniffing,
- tick paralysis,
- metabolic disturbances (hypokalaemia, hypophosphataemia)
Neuromuscular junction abnormalities
- Myasthenia gravis,
- botulism,
- organophosphate poisoning
- Muscular abnormalities
- Polymyositis,
- dermatomyositis,
- acute rhabdomyolysis
Investigations
CSF analysis: Protein elevated more than twice the normal (100-1000mg/dL) with no pleocytosis called albuminocytologic dissociation, glucose normal, bacterial cultures negative, rare isolation of specific viruses Transient increase in cell count (10-100/mm3) may occur but never sustained CSF protein- Often normal in first week but increased in more than 90% of patients at end of second week Investigations
Nerve conduction studies: motor nerve conduction velocity greatly reduced, sensory nerve conduction time is often slow Electrodiagnostic findings suggestive of GBS : an absent H reflex, low amplitude or absent sensory nerve action potentials(SNAP), reduced amplitudes of compound muscle action potentials (CMAPs), an abnormal F wave Later slowing of conduction velocity, conduction block
Antiganglioside antibodies against GM1 and GD1 are sometimes elevated according to variants of GBS Serological testing for campylobacter and helicobacter Investigations.. MRI : thickening of the cauda equina and intrathecal nerve roots with gadolinium enhancement. Findings are fairly sensitive and present in >90% of patients
Serum creatine kinase (CK) level- mildly elevated or normal
Muscle biopsy is not usually required, normal in early stage, later shows denervation atrophy
Sural nerve biopsy tissue shows segmental demyelination, focal inflammation, and wallerian degeneration
Variants of GBS
Acute inflammatory demyelinating polyradiculoneuropathy (AIDP)
Most common variant in Europe and USA, most studied type
Is triggered by antecedent viral or bacterial infection
Electrophysiologic findings demonstrate demyelination.
Remyelination occurs after the immune reaction stops
Acute motor axonal neuropathy (AMAN)
Pure motor axonal form of neuropathy
60% are seropositive for campylobacteriosis.
Electrophysiologic studies are normal in sensory nerves, reduced or absent in motor nerves.
Recovery is typically more rapid.
High proportion of pediatric patients
Acute motor sensory axonal neuropathy (AMSAN)
Wallerian-like degeneration of myelinated motor and sensory fibers
Minimal inflammation and demyelination
Typically affects adults
Miller Fisher syndrome
Rare disorder
Rapidly evolving ataxia, areflexia, mild limb weakness, and ophthalmoplegia
Sensory loss unusual, but proprioception may be impaired.
Demyelination and inflammation of cranial nerve III and VI, spinal ganglia, and peripheral nerves
Resolution occurs in one to three months.
Acute panautonomic neuropathy
Rarest of all the variants
Sympathetic, parasympathetic nervous systems are involved.
Cardiovascular involvement is common (postural hypotension, tachycardia, hypertension, dysrhythmias).
Recovery is gradual and often incomplete.
Often combined with sensory features
Variants of GBS..
Congenital Guillain-Barre syndrome is described rarely:
C/F : generalized hypotonia, weakness and areflexia in an affected neonate
Inves : Fulfilling all electrophysiologic and cerebrospinal fluid (CSF) criteria and in the absence of maternal neuromuscular disease.
Treatment: might not be required and there is gradual improvement over the first few months and no evidence of residual disease by 1 yr of age.
Diagnosis of Typical GBS
Required for Diagnosis
1. Progressive weakness in both arms & legs from mild paresis to complete paralysis
2. Generalized hypo- or areflexia
Strongly Supportive of Diagnosis
- Progression of symptoms from days to 4 weeks
- Relative symmetry of symptoms
- Mild sensory symptoms
- Cranial nerves involvements
- Autonomic dysfunction
- High CSF protein
- Typical electrodiagnostic features
Features that raise doubt about the diagnosis
- Severe pulmonary dysfunction with limited limb weakness
- Severe sensory signs with limited weakness
- Bladder or bowel dysfunction at onset
- Fever at onset
- Sharp sensory level
- Marked persistent asymmetry of weakness
- Persistent bladder dysfunction
- Increased number of mononuclear cells in CSF
Treatment
Treatment of GBS has two components:
Supportive care and Specific therapy.
Supportive care remains the cornerstone of therapy
In early stage of this acute disease patient should be admitted to hospital for observation because the ascending paralysis may rapidly involve respiratory muscles
Patients with slow progression may simply be observed for stabilization and spontaneous remission without treatment
Rapidly progressive ascending paralysis is treated with intravenous immunoglobulin IVIG
Each day counts after 1st motor symptom till 2 weeks, when treatment may be effectiveEither IvIg or Plasmapharesis, IvIg choosen for ease of administration & good saftey record GBS autoantibodies are neutralized by anti-idiotypic antibodies present in IVIg preparations Lack of noticeable improvement following IVIg or PE is not an indication to treat with the alternate one.In worsening case critical care monitoring for vital capacity, heart rhythm, blood pressure, nutrition, deep vein thrombosis prophylaxis, early consideration (after 2 weeks of intubation) for tracheostomy, and chest physiotherapySome may need temporary venous pacemaker Frequent turning, assiduous skin care, physiotherapy for joint contracture.
IVIG
Dose: 0.4 gm/kg/day for 5 consecutive days is commonly recommended protocol though duration of 2 and 3 days are also used Side effects- IVIg expands the plasma volume so it must be administered with caution in patients with congestive heart failure and renal insufficiency. Patients may develop fever, myalgia, headache, nausea, and vomiting, but these “influenza-like” symptoms are self-limiting. Patients also may develop aseptic meningitis, neutropenia, and hypertension. A history of previous anaphylaxis to IVIg is a contraindication to repeat treatment.
Plasmapharesis
Plasmapheresis (five exchanges over five to eight days)
The usual regimen is plasma exchange (PE) five times during 2 weeks, with a total exchange of about five plasma volumes.
Disadvantages of plasmapheresis include its rare complications, such as sepsis, that are believed to be caused by depletion of immunoglobulins. [Evidence level A, RCT] If fresh frozen plasma is used as replacement fluid, there is a risk of acquiring viral infections such as hepatitis and HIV.
Corticosteroids
Corticosteroids were once believed to be useful in the treatment of GBS because of its immune-mediated inflammatory mechanism. However, a Cochrane Database Review of randomized trials, which included 195 patients treated with corticosteroids compared with controls, showed no difference in outcome
Corticosteroids for GBS.. cochrane review
Six trials with 587 participants provided data for the primary outcome. According to moderate quality evidence, the disability grade change after four weeks in the corticosteroid groups was not significantly different from that in the control groups
In four trials of oral corticosteroids with 120 participants in total, there was significantly less improvement after four weeks with corticosteroids than without corticosteroids
Corticosteroids for GBS
According to moderate quality evidence, corticosteroids given alone do not significantly hasten recovery from GBS or affect the long-term outcome. According to low quality evidence oral corticosteroids delay recovery. Diabetes requiring insulin was significantly more and hypertension less common with corticosteroids.
Prognosis
85 % achieve full functional recovery
Mortality < 5% in optimal setting
Bad prognosis in – Severe proximal motor and sensory axonal damage, a fulminant or severe attack, delay in the onset of treatment (Harrisons 18th edition)
Cranial nerve involvement, intubation and maximum disability at the time of presentation (Nelson)
Electrophysiologic features of Conduction block have good prognosis
Morbidity and mortality in GBS
Improvement usually follows a gradient opposite the direction of involvement: bulbar function recovering first and lower extremity weakness resolving last.
Easy fatigue is one of the most common chronic symptoms
In epidemiologic surveys, the overall death rate related to GBS ranges from 2-12% of patients. GBS-associated mortality rates increase markedly with age. In the United States, the case-fatality ratio ranges from 0.7% among persons younger than 15 years to 8.6% among individuals older than 65 years. * http://emedicine.medscape.com/article/315632-overview
Pain in Guillain-Barre syndrome: a long-term follow-up study
This was a prospective cohort study in 156 patients with GBS (including 18 patients with Miller Fisher syndrome [MFS]
Pain was reported in the 2 weeks preceding weakness in 36% of patients, 66% reported pain in the acute phase (first 3 weeks after inclusion), and 38% reported pain after 1 year
Pain is a common and often severe symptom in the whole spectrum of GBS (including MFS, mildly affected, and pure motor patients). As it frequently occurs as the first symptom, but may even last for at least 1 year, pain in GBS requires full attention http://www.ncbi.nlm.nih.gov/pubmed/20861453 ,Neurology 2010 Oct 19;75(16):1439-47.
Pain and psychologic stress should be treated. Narcotics should be used with caution (as risk of ileus already present)
Physical therapy, including gentle massage, passive range-of-motion exercises, and frequent position changes may provide pain relief.
Carbamazepine (Tegretol) and gabapentin (Neurontin) have been used as adjuncts in pain management in GBS.
Patients treated with these medications (carbamazepine & Gabapentine) required less narcotic analgesia with fewer narcotic side effects and minimal sedation compared with those who received placebo.
Patients are paralyzed by the illness, but mentally alert and fearful. Reassurance and discussion about the phases of illness and recovery can help reduce psychologic stress.@
@Tripathi M, Kaushik S. Carbamazepine for pain management in Guillain-Barré syndrome patients in the intensive care unit. Crit Care Med. 2000;28:655–8.
Pandey CK, Bose N, Garg G, Singh N, Baronia A, Agarwal A, et al. Gabapentin for the treatment of pain in Guillain-Barré syndrome: a double-blind, placebo-controlled, crossover study. Anesth Analg. 2002;95:1719–23.
Future trends in GBS
Can we use a second course of IvIg in patients with bad prognosis in acute phase ….
Agents that interfere with complement activation can be attractive to be tested in the early phase of disease …. Eg : Eculizimab
Less aggressive treatment trials can be done in milder form of GBS ..
More attention should be paid to pain, autonomic dysfunction and severe fatigue.
References
Nelson textbook of pediatrics, 19th edition
Pediatrics in Review 2012;33;164 Beth A. Rosen
IVIG Treatment and Prognosis in Guillain–Barré Syndrome- J Clin Immunol (2010)
(American academy of neurology, official site)
www.cdc.gov
The lancet
Thank You !!!
- Dr Sujit Kumar Shrestha
- MD resident in Pediatrics
- TUTH, Nepal