Sickle Cell Disease: A Primer

Background:
Sickle cell disease (SCD) or sickle cell anemia (SCA) is a hemoglobinopathy wherein abnormal hemoglobin is produced. It was first noted by James Herrick a medical student of African descent in 1911, when he discovered sickle-shaped erythrocytes while examining a student’s blood. Linus Pauling further categorized the disorder by noting that when the haemoglobin from an individual with sickle cell disease was analyzed by allowing it to migrate or run in a gel, an abnormal hemoglobin electrophoretic pattern was seen. He designated this haemoglobin as hemoglobin S, while declaring SCD as a molecular disease.
by allowing it to migrate or run in a gel, an abnormal hemoglobin electrophoretic pattern was seen. He designated this haemoglobin as hemoglobin S, while declaring SCD as a molecular disease.
Sickle cell disease (SCD) affects millions of people worldwide, of which India is estimated to be home to over 50% of the world’s patients with SCD. It is highly prevalent in central India and among the tribal belts in western, eastern and southern India. It has been estimated that over 5000 babies with sickle cell disease would be born each year in India
Molecular basis
Hemoglobin is produced by genes that control the expression of the hemoglobin protein. So the α –globin gene and the β globin gene produce the α and β globin molecules respectively, 2 molecules of each will then combine to form a tetrameric structure, with the addition of a haem group (iron molecule) to become a functional unit. In case of sickle cell disease there is a mutation (change in the genetic sequence) in the β -globin gene, the nucleotide Adenosine gets changed to Thymine. This results in change in the amino acid sequence where Glutamic Acid is replaced by Valine at the sixth position of the beta-globin chain glutamic (GAG) —> valine(GTG). Hence SCD is called as a monogenic disorder because the disease occurs due to change in a single gene.
Functional Consequences
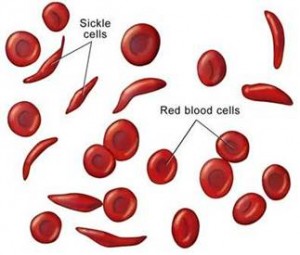
Because of the change in the amino acid sequence, this abnormal Hb designated as HbS polymerizes or forms rope-like fibre during deoxygenation (remember the RBCs have to give the oxygen that they are carrying from the lungs to the cells of our body, a process called deoxygenation). As the deoxy-HbS polymerizes, fibers are formed which align, the erythrocyte is transformed into a “sickle” shape or in other words there is sickling. The normal structure of erythrocytes or RBCs (red blood cells) is a biconcave disc, which is very critical to its function of supplying oxygen to all cells of the body. This structure ensures that the RBCs are deformable or flexible, that is they can pass through narrow capillaries which are much smaller than its own diameter of 7.2 micron and again regain back its shape. In contrast the sickle shaped cells are not deformable or inflexible and get stuck in such narrow capillaries (vascular blockage) leading to clinical consequences.
Clinical Consequences
The inflexible red cells cannot pass through the microcirculation efficiently, and thereby induce ischemia (or death) in tissues distal to the vascular blockage and results in anemia (due to destruction of the red cells) and intermittent vaso-occlusion causing tissue damage and pain. Although all patients with SCD have exactly the same molecular defect, there is considerable clinical variation, ranging from death in early childhood to a normal life span with few complications. In fact several studies are now ongoing to look at modifier genes which could ameliorate the disease severity.  Other clinical complications include
Other clinical complications include
• Acute chest syndrome: It is a non-infectious vaso-occlusive crisis of the pulmonary vasculature commonly seen in patients with sickle cell anemia. The crisis is often initiated by a lung infection, and the resulting inflammation and loss of oxygen tension leads to sickling of red cells and further vasoocclusion.
• Stroke: It is a devastating complication of SCD, which occurs in about 11% of patients under 20 years of age. A stroke, or cerebrovascular accident (CVA), is the rapid loss of brain function(s) due to disturbance in the blood supply to the brain. This can be due to ischemia (lack of blood flow) caused by blockage (thrombosis, arterial embolism), or a hemorrhage. As a result, the affected area of the brain cannot function, which might result in an inability to move one or more limbs on one side of the body, inability to understand or formulate speech, or an inability to see one side of the visual field .
• Splenic and renal dysfunction : Improper functioning of spleen and kidney.
• Pain crises.
• Leg ulcers : A cutaneous discontinuity seen on the legs of SCD patients.
• Priapism : It is a persistent, usually painful, erection that lasts for more than four hours and occurs without sexual stimulation, occurs in 30%–45% of male patients with SCD.
• Osteonecrosis : It is bone death caused by poor blood supply to the area. It is most common in the hip and shoulder, but can affect other large joints such as knee, elbow, wrist and ankle and ;
• Susceptibility to bacterial infections: Infection and bacteremia are common in SCD and severe bacterial infections are the major causes of morbidity and mortality in SCA.
Also Read –Why Salmonella Osteomyelitis is common in SCD?
Mode of Inheritance: Sickle Cell Disease is an autosomal recessive disease, which means that the disease is manifested only if both the genes inherited are defective. We inherit one set of chromosome (i.e. large chunk of DNA which houses the gene and is present in all our cells) from both our parents we have copies of each gene (though there are some exceptions). So only if both the copies of gene have the sickle cell disease mutation the individual manifests the disease. Such individuals are homozygous for the mutation and suffer from sickle-cell anaemia also referred to as “HbSS”, “SS disease”, “haemoglobin S” or permutations of those names If only one defective copy is received then the person has sickle cell trait and does not exhibit any of the symptoms. Such individuals are heterozygous and the condition is referred to as “HBAS” or “sickle cell trait”. However such a person can pass this genetic defect to his progeny and hence genetic counseling is recommended whenever any person has tested positive in any of the tests for SCD.
Diagnostic Tests:
The first line tests whenever there is a suspicion of hemoglobinopathies incuding SCD would be CBC (complete blood count), and blood film. Presence of crescent shaped RBCs instead of the regular doughnut shape is first indication of SCD. In HbSS, the full blood count reveals haemoglobin levels in the range of 6–8 g/dL with a high reticulocyte count (as the bone marrow compensates for the destruction of sickle cells by producing more red blood cells). In other forms of sickle-cell disease, Hb levels tend to be higher.
 In a low resource testing Solubility Test is often performed to screen for such individuals. The solubility test is based on low solubility of reduced sickle haemoglobin in reducing solution such as (sodium dithionite). Primary screening tests include a combination of electrophoretic and chromatographic methods. Abnormal haemoglobin forms can be detected on haemoglobin electrophoresis, a form of gel electrophoresis on which the various types of haemoglobin move at varying speeds. Sickle-cell haemoglobin (HbS) and haemoglobin C with sickling (HgbSC)—the two most common forms—can be identified from there.The diagnosis can be confirmed with high-performance liquid chromatography (HPLC).
In a low resource testing Solubility Test is often performed to screen for such individuals. The solubility test is based on low solubility of reduced sickle haemoglobin in reducing solution such as (sodium dithionite). Primary screening tests include a combination of electrophoretic and chromatographic methods. Abnormal haemoglobin forms can be detected on haemoglobin electrophoresis, a form of gel electrophoresis on which the various types of haemoglobin move at varying speeds. Sickle-cell haemoglobin (HbS) and haemoglobin C with sickling (HgbSC)—the two most common forms—can be identified from there.The diagnosis can be confirmed with high-performance liquid chromatography (HPLC).
Molecular Diagnosis using the polymerase chain technology (PCR) can be done to confirm the results of HPLC and to distinguish between the homozygous (HBSS) and heterozygous trait (HBAS).
Pre-implantation Genetic Diagnosis (PIGD) is an option for couples who are both carriers of the sickle cell trait but who do not want to give birth to a child with sickle cell anaemia. PIGD involves removing eggs from a woman’s ovaries. These are fertilised using sperm taken from her partner. The fertilised embryo can be tested for sickle cell anaemia. If test results are negative, the embryo can then be implanted into the woman’s womb.
Treatment:
Sickle cell anaemia has no widely available cure. However, treatments can help relieve symptoms and treat complications. The goals of treating sickle cell anaemia are to relieve pain; prevent infections, organ damage, and strokes; and control complications (if they occur). Infants who have been diagnosed with sickle cell anaemia through newborn screening are treated with antibiotics to prevent infections and receive needed vaccinations.
Hydroxyurea treatment: Fetal Hemoglobin designated as HbF is a type of hemoglobin whose concentration is high at birth, it is produced by the alpha α and gamma globin genes and then at around 4-6 months adult hemoglobin starts predominating which is produced by alpha α and beta globin genes. The concentration of HbF in adults was seen to vary, but what was clearly seen was that SCD patients who had higher HbF concentration experienced reduced rate of acute painful episodes, fewer leg ulcers, less osteonecrosis, less frequent acute chest syndromes, and reduced disease severity. HbF has an ameliorative effect on SCD patients because the gamma-chains of HbF do not polymerize with HbS. To date, the only drug that is therapeutically used in this way is the S-phase specific chemotherapeutic agent, hydroxyurea (HU) which increases HbF production.
To summarize SCD is a debilitating monogenic disease with variable clinical phenotypes. Currently several clinical trials are ongoing to utilize gene therapy for overcoming this disease. Research is focused on understanding what factors both genetic and environmental can ameliorate the disease severity. Considering the high prevalence of SCD in India, a Sickle Cell Institute has been commissioned in Raipur, Chattisgarh to conduct research, screen as well as counsel patients and family members of affected individuals.
About the Author-
Dr. Aparna Bhanushali,
Research Scientist,
SRL R&D