Developmental and Acquired Anomalies in Bone Cells, Matrix and Structure

Biochemically, bone is composed of inorganic elements (65%) and organic elements (35%).
A. Inorganic component: Mineral “calcium hydroxyapatite [10Ca:6(PO4):(OH)3]”. Bone that is unmineralized is known as “osteid”.
B. Organic component: Bone cells and matrix proteins
a. Bone forming cells:
i. Osteoprogenitor cells:
– pluripotent mesenchymal stem cell
– present in the inner portion of the periosteum, in the endosteum, and in the central canal of compact bone
– stimulated by TGFβ for differentiation into osteoblast.
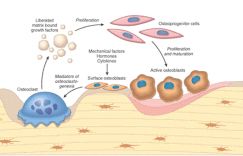
ii. Osteoblasts:
– synthesize, transport and arrange matrix proteins
– initiate mineralization process
– express cytokine, hormone binding receptors.
– Under the control of calcitonin to take blood calcium and put it into the bone.
iii. Osteocytes:
– Osteoblasts surrounded by matrix after end of their life span
– Osteocytes : Osteoblasts ratio=10:1
b. Bone resorbing cells (Osteoclasts):
– Macrophage lineage cells
– IL-1, IL-3, IL-6, IL-11, TNF, GM-CSF and M-CSF for osteoclast differentiation and maturation
– Osteoclastogenesis: Differentiation of preosteoclasts into osteoclasts by RANK-RANKL contact
– Osteoprotegrin (OPG) inhibits osteoclastogenesis by binding to RANKL
– Scalloped resorption pits osteoclasts produce and reside in are known as “Howship lacunae”
– Under control of PTH to chew up the calcium of bone and put it into blood
c. Bone Proteins:
– Type I collagen (90%)
– Cell adhesion proteins
– Clacium binding proteins
– Mineralization proteins (Osteocalcin)
– Enzymes (collagenase, alkaline phosphatase)
– Cytokines (Prostaglandins, IL-1, IL-6, RANKL)
– Growth factors (GF-1, TGF-β, PDGF)
Osteoblasts and osteoclasts act in coordination and are considered to be functional unit of bone known as the Basic Multicellular Unit (BMU).
ANATOMY OF A LONG BONE:
The bone is enclosed in a tough, fibrous, connective tissue covering called the periosteum, which is continuous with the ligaments and tendons that anchor bones. The periosteum contains blood vessels that enter the bone and service its cells. At both ends of a long bone is an expanded portion called an epiphysis; the portion between the epiphyses is called the diaphysis. The diaphysis is not solid but has a medullary cavity containing yellow marrow. The medullary cavity is bounded at the sides by compact bone. The epiphyses contain spongy bone. Beyond the spongy bone is a thin shell of compact bone and, finally, a layer of hyaline cartilage called the articular cartilage. The medullary cavity and the spaces of spongy bone are lined with endosteum, a thin, fibrous membrane.
i. Cortical (80%):
ii. Spongy/Trabecular/Cancellous (20%):
SPONGIOSA:
i. Primary spongiosa : the mineralized cartilage in the developing metaphysis.
ii. Secondary spongiosa : second stage in the mineralization of bony trabeculae; comprises the enlarged, mineralized bony trabeculae of the primary spongiosa.
DEVELOPMENTAL & ACQUIRED ABNORMALITIES IN BONE CELLS, MATRIX AND STRUCTURE:
Dysostoses: Developmental anomalies resulting from localized problems in the migration of mesenchymal cells and their formation of condensation.
Dysplasias: Mutations in the regulators of organogenesis (growth factors, receptors, matrix components) affecting cartilage and bone tissues globally.
A. Defect in nuclear proteins and transcription factors: usually due to mutation in homeobox genes
i. Congenital absence of phalanx, rib or clavicle
ii. Polydactyly
iii. Syndactyly
iv. Craniorachischis
B. Defects in hormones and signal transducing mechanisms:
i. Achondroplasia :
– Mutation in the FGFR3 coding genes causing constant activation of FGF3 leading to growth suppression cartilage proliferation
– Autosomal dominant disorder
– Non-lethal dwarfism
ii. Thanatophoric dwarfism: lethal
Some common features in both:
a. Shortening of limbs
b. Frontal bossing
C. Defects in extracellular structural proteins:
i. Osteogenesis Imerfecta:
– Deficiencies in the synthesis of type I collagen
– Mutations in the gene that code for a1 and a2 chains of type I collagen
– 4 variants: type I, type II, type III, type IV
– Type II: Fatal in utero or perinatal period
– Other types are compatible with survival
– Features:
a. Blue sclera
b. Hearing loss
c. Dental imperfections
d. Skeletal fragility
ii. Achondrogenesis II, Hypochondrogenesis, Sickler syndrome: collagen II
iii. Multiple epiphyseal dysplasia: collagen IX
iv. Schmid metaphyseal chondrodysplasia: collagen X
D. Defects in misfolding and degradation of macromolecules:
i. Mucopolysaccharidoses:
– A lysosomal storage disease
– Deficiency in enzymes that degrade dermatan, heparain and keratain sulfate
– Cartilage disorders: Short stature, chest wall abnormalities, malformed bones
E. Defect in metabolic pathways (Enzymes, Ion Channels, and Transporters):
i. Osteopetrosis:
– Reduced osteoclast bone resorption
– Known as marble bone disease: brittle and bones fracture like chalk
– 4 variants: infantile malignant, type II carbonic anhydrase deficiency and autosomal dominant type I and II
– Osteoclasts cannot acidify the resorption pits preventing digestion of bone
– Ends of the long bone are bulbous (Erlenmeyer flask deformity)
– Primary spongiosa persists and fills the medullary cavity
– Brittle bone, fracture, hepatosplenomegaly and anemia, nerve compressions
F. Diseases associated with decreased bone mass:
i. Osteoporosis:
– Accelerated bone loss causing increased porosity of skeleton
– Peak bone mass achieved during early adulthood
– Factors leading to osteoporosis:
a. Age related (Senile) : Decreased replicative activity of osteoprogenitor cells, Decreased synthetic activity of osteoblasts, Decreased biologic activity of matrix-bound growth factors, Reduced physical activity (Wolff’s law – a bone is only big and strong as it has to be)
b. Post-menopausal: Decreased serum estrogen Increased IL-1, IL-6, TNF levels Increased expression of RANK and RANKL Increased osteoclastic activity
c. Genetic: Polymorphism in vitamin D and PTH receptor molecules
d. Nutrition: Calcium intake in diet
e. Secondary factors: hyperparathyroidism, multiple myeloma, malnutrition, vitamin C and D deficiencies, corticosteroid administration, anemia, osteogenesis imperfect, immobilization, etc.
– Features: Fractures, pain, lumbar lordosis, kyphoscoliosis
– Treatment and prevention: Exercise, Calcium & vit.D intake, estrogen replacement, etc.
G. Disease caused by Osteoclast dysfunction:
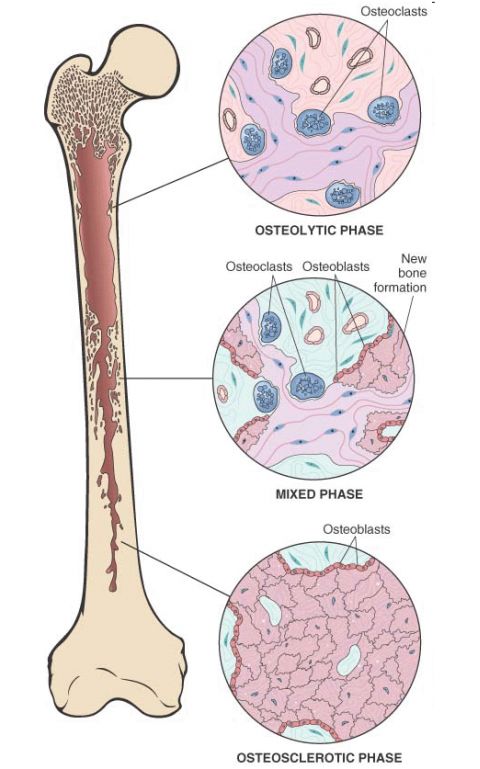
i. Paget Disease (Osteitis Deformans):
– Collage of matrix madness (osteoclast gone wild); osteoclasts are hyperresponsive to RANKL and vitamin D
– Caused due to a slow virus infection by a paramyxovirus which induces production of IL-6, M-CSF and other cytokines which stimulate osteoclastic activity
– 3 stages:
a. Osteolytic stage: increased resorption pits and osteoclastic activity
b. Mixed osteoclastic-osteoblastic stage: osteoclastic activity persists but osteoblastic activity dominates leading to formation of woven or lamellar bone
c. Osteosclerotic stage: burn out quiescent stage; soft and thickened trabeculae and cortices
– Histologic hallmark: Mosaic pattern, Jigsaw like lamellar bone
– Clinical findings:
a. Elevated alkaline phosphate and urine hydroxyproline
b. Monostotic (15%) and Polyostotic (85%)
c. Pain due to microfactures (chalk like fractures) and nerve compression
d. Leontiasis osseas and platybasia
e. Kyphosis
f. Heart diseases
g. Progression to osteoporosis and sarcomas like osteosarcoma and chondrosarcoma
H. Diseases associated with abnormal mineral homeostasis:
a. Rickets and Osteomalacia:
– Lack of vitamin D or defect in its metabolism
– Rickets (osteopenia usually not seen) in children and osteomalacia in adults (characterized by osteopenia)
b. Hyperparathyroidism:
– Primary hyperparathyroidism due to adenoma of parathyroid gland
– Secondary hyperparathyroidism due to hypocalcemia is not as severe as primary one
– Entire skeleton is affected ; fractures
– Osteitis fibrosa cystica (von Recklinghausen’s disease of bone) in severe cases
– Brown tumor due to increased vascularity, hemorrhage and hemosiderin deposition
– Dissecting osteitis, osteopenia, thinning of cortices
c. Renal osteodystrophy:
– Skeletal changes of chronic renal disease like osteitis fibrous cystica, osteomalacia, osteosclerosis, osteoporosis, growth retardation, etc.
– Pathogenesis:
a. Phosphate retention and hyperphosphatemia
b. Hypocalcemia (vitamin D : 1,25(OH)2D3 deficiency
c. Increased PTH
d. Metabolic acidosis Release of hydroxyapatites from matrix
e. Iron accumulation of bone and aluminium deposition at site of mineralization
– Complication: Amyloidosis


1 Comment
Very informative article… Looking forward for more articles on your blog
Comments are closed.